News
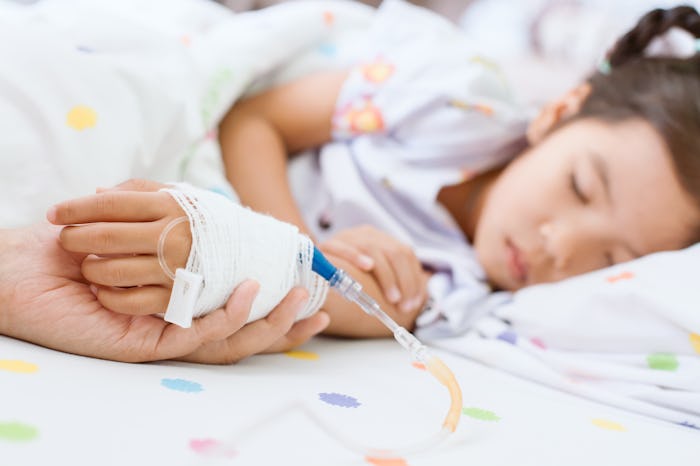
FDA Gives Full Approval For First Covid-19 Treatment For Young Children
The FDA warns that the approval is not a substitute for vaccination when possible.
On April 25, the U.S. Food and Drug Administration (FDA) granted full approval of remdesivir for the treatment of young children with Covid-19. Patients over the age of 28 days old who are hospitalized with the virus — or who are ill and at higher risk of hospitalization — can now be treated with the same drug that has proven effective in adults.
“As COVID-19 can cause severe illness in children, some of whom do not currently have a vaccination option, there continues to be a need for safe and effective COVID-19 treatment options for this population,” Patrizia Cavazzoni, M.D., director of the FDA’s Center for Drug Evaluation and Research said in a statement issued by the FDA. “Today’s approval of the first COVID-19 therapeutic for this population demonstrates the agency’s commitment to that need.”
Remdesivir, officially marketed and sold in the U.S. as Velkury, is produced by Gilead Sciences and was the first drug approved for the treatment of Covid back in October 2020, but only for patients over the age of 12. Based on the results of clinical trials of remdesivir in adults and children, it is now the first approved Covid-19 treatment for children under the age of 12.
Remdesivir does not cure Covid, but rather works by interrupting the production of the virus. It does this by interfering with one of the key enzymes the virus needs to replicate RNA. Currently, the only approved dosage form is Veklury for injection, which must be administered via intravenous infusion.
The FDA cautions, however that this treatment, while effective against serious illness, is no substitute for vaccination. Vaccination is currently available for children over the age of 5, for free, even without insurance, and regardless of immigration status. Vaccines for children under the age of 5 were initially expected in early 2022, but researchers found that the two doses they had tested were not strong enough to protect against new Covid variants like Omicron. Now researchers at Pfizer are waiting for data on a three-dose vaccination for that age group. Emergency Use Authorization (EUA), according to reporting from CNN, is expected Summer 2022. While children tend to be less seriously affected by the virus, serious illness and death have occurred.
While many parents of very young children are still anxiously awaiting Emergency Use Authorization (EUA) approval for a vaccine for toddlers and infants, effective treatment for the most vulnerable is a positive development.